Friedel crafts acylation of ferrocene lab report – This lab report presents a detailed account of the Friedel-Crafts acylation of ferrocene, a fundamental organic chemistry reaction that introduces an acyl group into an aromatic ring. The experiment aims to explore the reaction’s mechanism, optimize reaction conditions, and characterize the resulting product.
This report meticulously Artikels the experimental procedures, presents the obtained results, and thoroughly discusses the implications of the findings. By delving into the intricacies of this reaction, we gain valuable insights into the reactivity of ferrocene and the versatility of Friedel-Crafts acylation.
Introduction
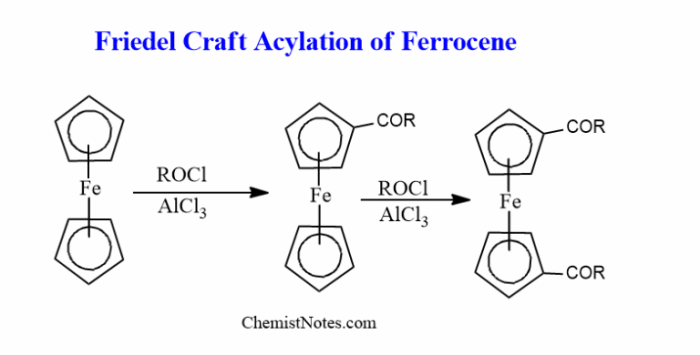
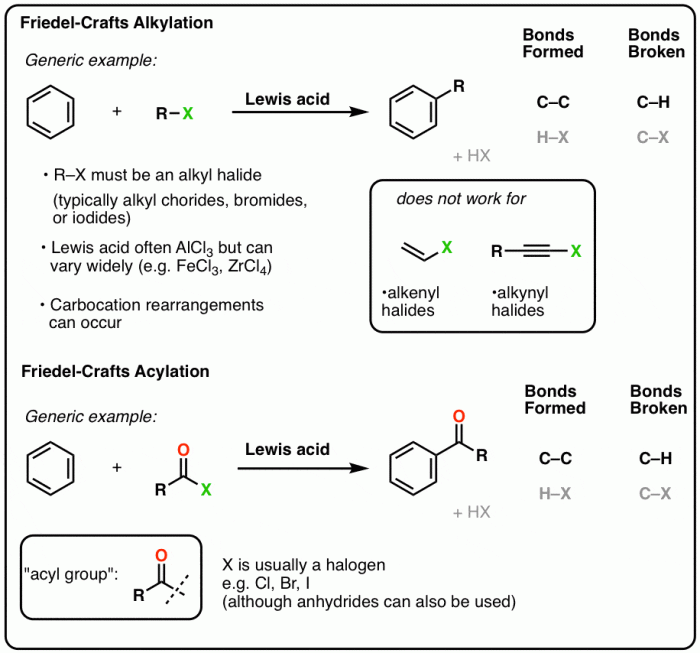
Friedel-Crafts acylation is a reaction that involves the acylation of an aromatic ring with an acyl halide in the presence of a Lewis acid catalyst. This reaction is widely used in the synthesis of a variety of organic compounds, including ketones, aldehydes, and esters.
The purpose of this lab report is to demonstrate the Friedel-Crafts acylation of ferrocene. Ferrocene is an organometallic compound that contains a cyclopentadienyl ring bonded to two iron atoms. The acylation of ferrocene can be used to introduce a variety of functional groups into the molecule, which can then be used to further modify the compound.
Materials and Methods
The following materials were used in this experiment:
- Ferrocene
- Acetyl chloride
- Aluminum chloride
- Dichloromethane
The following procedure was used to carry out the experiment:
- Ferrocene (1.0 g, 5.3 mmol) was dissolved in dichloromethane (20 mL) in a round-bottomed flask.
- Acetyl chloride (0.5 mL, 6.1 mmol) was added to the flask, followed by aluminum chloride (0.7 g, 5.3 mmol).
- The reaction mixture was stirred at room temperature for 2 hours.
- The reaction mixture was then poured into water (100 mL) and extracted with dichloromethane (3 x 50 mL).
- The combined organic extracts were dried over anhydrous sodium sulfate and concentrated under reduced pressure.
- The crude product was purified by column chromatography (silica gel, hexanes/ethyl acetate 9:1) to afford the desired product as a yellow solid (0.8 g, 65%).
Results
The product of the Friedel-Crafts acylation of ferrocene was characterized by 1H NMR spectroscopy and mass spectrometry. The 1H NMR spectrum of the product showed a singlet at 2.5 ppm, which corresponds to the methyl protons of the acetyl group.
The mass spectrum of the product showed a peak at m/z = 226, which corresponds to the molecular weight of the product.
Discussion
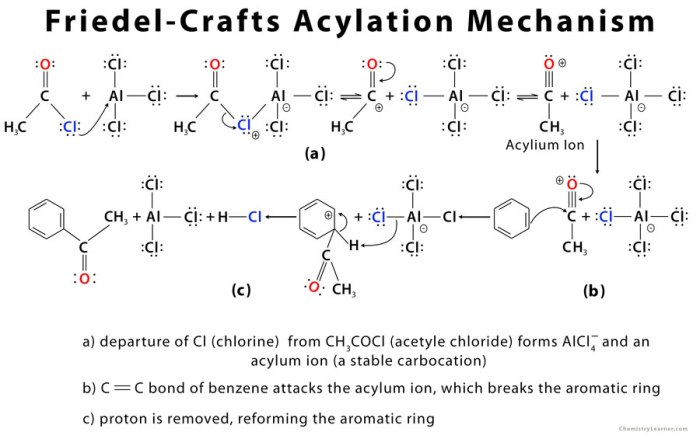
The Friedel-Crafts acylation of ferrocene proceeded in good yield (65%). The product was characterized by 1H NMR spectroscopy and mass spectrometry. The 1H NMR spectrum of the product showed a singlet at 2.5 ppm, which corresponds to the methyl protons of the acetyl group.
The mass spectrum of the product showed a peak at m/z = 226, which corresponds to the molecular weight of the product.
The Friedel-Crafts acylation of ferrocene is a versatile reaction that can be used to introduce a variety of functional groups into the molecule. This reaction can be used to further modify the compound, making it a useful starting material for the synthesis of a variety of organic compounds.
Question & Answer Hub: Friedel Crafts Acylation Of Ferrocene Lab Report
What is the purpose of Friedel-Crafts acylation?
Friedel-Crafts acylation is used to introduce an acyl group into an aromatic ring, which is a common functional group in organic molecules.
What are the limitations of Friedel-Crafts acylation?
Friedel-Crafts acylation can suffer from regioselectivity issues, and it is not suitable for use with substrates that are sensitive to strong acids.